15-10-2025
From Planning to Execution: Your All-In-One Cardiology Product Launch Checklist
Launching a new cardiology product is no small feat. It’s a journey that demands precision, collaboration, and a deep understanding of the science behind them and the market.
From early-stage planning to post-launch execution, every step plays a crucial role in ensuring success. This is why having a clear and structured product launch strategy is essential to avoid common missteps and ensure success.
Medical affairs has long shifted from a support function to a strategic, critical central role in launch planning — bridging clinical, regulatory, market access, and commercial teams. This shift has been driven by stringent compliance requirements, a broader set of stakeholders involved, and their rising expectations.
This all-in-one guide breaks down the cardiology product launch process into a practical, easy-to-follow checklist. Whether you are preparing for regulatory milestones, crafting a market access strategy, or planning stakeholder engagement, you’ll find actionable steps to keep your team aligned with overall launch strategy goals.
A successful cardiology product, device, or drug launch strategy begins with a deep understanding of the therapeutic landscape and identifying unmet needs. More importantly, the role of medical affairs in pharmaceutical launch strategy cannot be understated.
Their involvement must start as early as 5 years, working towards the Target Product Profile (TPP) that can help shape medical strategy from the get-go. This early engagement ensures that unmet patient needs, clinical endpoints, payer requirements, and stakeholder expectations are embedded deeply in the product strategy.
This means deciding what you want to achieve for patients and the healthcare system, who needs to be informed, and how you’ll know if it’s working.
Navigating the regulatory environment is one of the most crucial elements of any pharma launch strategy. The stakes are extremely high, and even minor oversights in compliance can delay approvals and jeopardize credibility. This phase ensures that your product meets the stringent safety, efficacy, and ethical standards before entering the market.
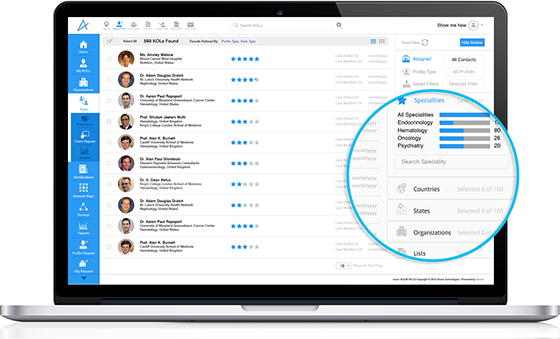
Engaging cardiology experts early is crucial to establishing scientific credibility, shaping product narratives, and ensuring a smoother post-launch adoption. At the heart of this phase is the identification and engagement of Key Opinion Leaders (KOLs). The influence of these experts can accelerate product awareness and adoption among Healthcare Professionals (HCPs).
Designing marketing strategies to launch a new product requires demonstrating both clinical impact and economic value. In cardiology, treatments are often high-cost (e.g., implantable devices), so securing reimbursement is critical to aid product adoption. Payers, hospitals, and insurers all want evidence that your product/solution works clinically and delivers patient-outcome benefits.
A thoughtful pricing and access strategy ensures your product is positioned as clinically and financially viable.
Commercial readiness is where scientific excellence meets market execution. Even with strong trial data and regulatory approvals, a cardiology product launch can falter if commercial teams are not fully prepared to communicate its value clearly and persuasively.
At this stage, organizations must ensure that sales, marketing, and medical affairs teams are aligned on a unified narrative. Training commercial teams in cardiology-specific language, treatment guidelines, and competitive positioning can help them confidently engage with highly knowledgeable HCPs.
The most innovative products or therapies can only succeed if they reliably reach the right hospitals, clinics, and patients. Distribution also involves designing a channel strategy that ensures availability across diverse care settings, ranging from large hospitals to regional clinics.
Another critical element is partnerships with distributor networks. Collaborating with established medical distributors can streamline access to high-demand cardiology products.
This is the moment when months of preparation finally meet the real-world market. In cardiology, the stakes are incredibly high, so launch execution means that the product is introduced in a way that inspires trust and encourages adoption among HCPs, payers, and patients.
Real-time monitoring of the launch campaign is equally important; it ensures agility. Adoption trends may vary across geographies, so launch teams must be prepared to adjust campaigns, scale distribution, or shift focus quickly. This flexibility is what separates strong launches from stalled ones.
A successful cardiology launch does not end with the product hitting the market; it begins a long-term cycle of optimization. Post-launch strategies are about sustaining momentum and staying ahead of competitors.
The primary focus is on collecting real-world evidence (RWE). While clinical trials demonstrate efficacy in controlled environments. However, payers and clinicians are increasingly interested in knowing how the product performs in broader, real-life patient populations.
Equally important is tracking key performance indicators (KPIs) such as prescription trends, hospital adoption rates, and geographical penetration, among others. Beyond quantitative metrics, qualitative indicators of assessing launch excellence include assessing HCP sentiment, evaluating payer engagement success, and tracking the success of scientific content.
These combined measures reveal where uptake is strong, where barriers exist, and which stakeholder group requires further engagement.
Glossary of Terms
- KOL - Key Opinion Leader
- HCP - Healthcare Professional
- DOL - Digital Opinion Leader
- HEOR - Health Economics & Outcomes Research
- KPI - Key Performance Indicator
- RWE - Real-world Evidence
- TPP - Target Product Profile