25 Sep 2023
Elevating Biopharmaceutical Clinical Trials Through Advanced Analytics
For the biopharmaceutical industry, developing new drugs and therapies is a complex and expensive endeavor. Clinical trials, which are the building blocks of this process, provide the necessary evidence for regulatory approval and eventual patient use.
However, the traditional clinical trial model comes with its own share of challenges. What constitutes these trials, what are the challenges in the traditional model, and how is advanced analytics reshaping the trials in the Life Sciences landscape? Let's explore further.
In this article:
Understanding Biopharmaceutical Clinical Trials
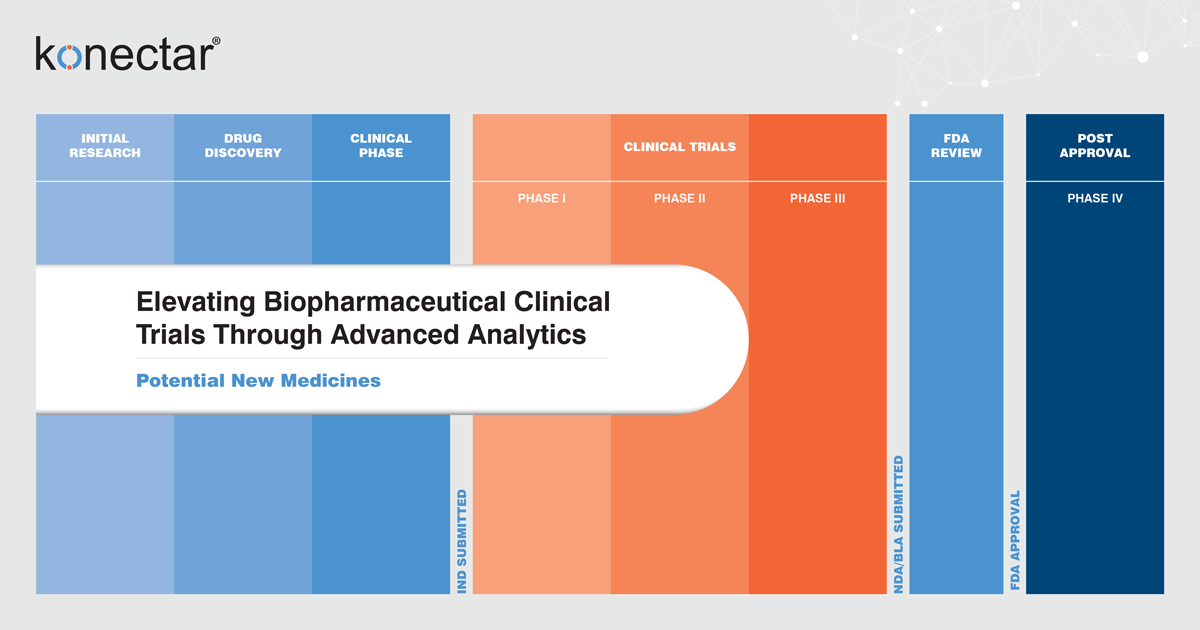
Before delving into how biopharmaceuticals are leveraging the power of analytics in clinical trials, let us first understand what constitutes them. These trials are rigorous and systematic processes to analyze the efficacy and safety of new drugs, therapies, or medical interventions before they can be approved for widespread use. These trials typically progress through three main phases:
Phase I trials primarily focus on evaluating the safety and establishing the appropriate dosage range of a new drug in a relatively small group of healthy volunteers. During this phase, researchers examine the drug's pharmacokinetics, including its absorption, distribution, metabolism, and excretion within the human body. This crucial information is obtained through the collection of blood samples, typically conducted in an inpatient setting, to monitor the drug's concentration in the bloodstream.
Phase II trials involve a more significant number of volunteers, which is used to assess the effectiveness of an experimental drug on a specific disease. These trials are typically randomized, where participants are assigned randomly to receive the experimental drug, a standard treatment, or a placebo. In double-blind studies, participants and KOLs (engaged in drug trial development) are unaware of the treatment to prevent bias, while in single-blind studies, only participants remain unaware.
Phase II trials offer a larger pool of patients, enabling better side effect observation, and around 33 percent of drugs progressing through Phases I and II move on to Phase III.
Phase III trials involve hundreds to even thousands of patients across multiple centers to assess a drug's safety and efficacy. Phase III studies can span several years, and upon completing a Phase III study, a life science company can file a drug application with the appropriate regulatory authorities to seek approval to market the drug.
However, traditional trials, despite their significance in advancing medical knowledge, frequently encounter a set of distinctive challenges, which necessitate innovative solutions to ensure the efficacy and efficiency of drug development.
Key Challenges Faced in Traditional Trials
-
Slow Patient Recruitment
Identifying and enrolling eligible patients who meet the strict criteria for a clinical trial can be time-consuming and arduous. It often involves screening a large number of potential participants to find those who fit the trial's specific requirements. Slow recruitment can significantly prolong the duration of the trial, delaying the availability of the treatment to the target population.
-
High Costs
The high financial burden associated with medical trials can hinder the ability of researchers and organizations to conduct tests, potentially resulting in fewer innovative treatments entering the biopharmaceutical market. These costs can also affect the pricing of the final drug or therapy, impacting patient access and affordability.
-
Regulatory Hurdles
Meeting regulatory requirements, obtaining approvals, and navigating the intricate regulatory landscape can be daunting and time-consuming. Delays in regulatory approvals can hinder the progress of trials, leading to missed opportunities for patients to access potentially life-saving treatments.
-
Data Management Issues
Clinical trials generate vast amounts of patient data, including medical records, test results, and adverse event reports. Managing and analyzing this data accurately and securely can be a complex task prone to errors. Data management issues can compromise the quality and integrity of trial results. Errors or data breaches can lead to regulatory compliance problems and erode trust in the trial's outcomes.
How Biopharmaceutical Companies Can Leverage Advanced Analytics?
Biopharma companies can utilize advanced analytics and technological advancements to mitigate some of these challenges. Clinical trial analytics employs machine learning algorithms to extract fresh insights from the vast healthcare wealth of data generated during the trials. Here's how life research companies can leverage advanced analytics to address key challenges in clinical trial
-
Patient Enrollment Distribution
Clinical trial analytics empowers companies to gain invaluable insights into the distribution of enrolled patients across diverse trials. This information is not only instrumental for medical teams, including Medical Affairs, in understanding the geographical reach of tests but also aids in identifying areas where specific medical conditions may be more prevalent. Such insights enable life sciences companies and researchers to tailor their recruitment strategies, ensuring the inclusion of a diverse and representative patient population.
-
Participant Demographics
Clinical trial analytics delves into the demographics of trial participants, shedding light on factors such as age, gender, ethnicity, and socioeconomic status. By analyzing this Healthcare Data, researchers can assess whether trial populations accurately reflect the broader patient population that will ultimately benefit from the treatment.
-
Adverse Event Frequencies
Understanding adverse events is fundamental in assessing the risks and benefits of new therapies. The analysis provides a detailed account of adverse event frequencies for completed trials and biopharmaceutical products. This data aids in evaluating the tolerability of treatments, identifying potential side effects, and refining patient care strategies. By quantifying adverse event rates, life sciences companies can enhance product safety profiles and develop comprehensive risk management plans.
-
Streamlining Process and Cost Savings
By employing AI-driven automation, processes that once required significant manual effort and time-consuming tasks can now be streamlined and executed more precisely. This helps in expediting medical trials while also making them more cost-effective. Leveraging data-driven insights can thus significantly enhance the life sciences companies' ability to meet and exceed these expectations.
FAQs
-
What are biopharmaceutical clinical trials?
These are the studies conducted to test new drugs and treatments to ensure they are safe and effective before they are used by patients.
-
How does advanced analytics help in these trials?
Advanced analytics, like AI and machine learning, can improve trials by making them faster, more efficient, and less costly.
-
What are some common issues in traditional biopharma clinical trials?
Common challenges include slow patient recruitment, high costs, regulatory hurdles, and managing large amounts of data.
-
Are there rules and ethics in using advanced analytics for clinical trials?
There are rules to protect patient data and ensure trials are conducted ethically.
-
What's the future of advanced analytics in biopharmaceutical clinical trials?
In the future, we can expect more AI-driven drug discoveries, personalized medicine, and better collaboration among researchers.
Key takeaways
The strategic integration of advanced analytics represents a critical turning point for biopharmaceutical companies, offering them a powerful means to overcome the complex challenges inherent in drug development. By leveraging sophisticated data analytics techniques and fostering robust HCP Connections, these companies can more effectively interpret vast amounts of information generated during clinical trials and research processes. This will expedite decision-making and accelerate the timeline for bringing new therapies to patients in need.